How Does The LiB Work?
It has been almost three decades that the Lithium-ion Battery (LiB) was first commercialized in 1991 by Sony (I have worked at the same R&D lab! 😊). LiBs has shown good reliability over time and now it is the most sought after energy storage chemistry for automotive and numerous other consumer applications.
In this blog post, I will describe a fundamental working mechanism of a LiB. A large number of resources are available on the internet for getting insight into the LiB mechanism. I will try to give a consolidated and brief overview of:
- LiB charge-discharge mechanism
- Non-idealities in LiB operation
Overview of Energy Storage Devices
Figure 1 shows different types of energy storage mechanisms ranging from mechanical, electrical and chemical energy storage (it is not an all-inclusive picture. Numerous other techniques exist for storing different forms of energies) Out of those main three types, Chemical energy storage devices are called 'Batteries' in general.
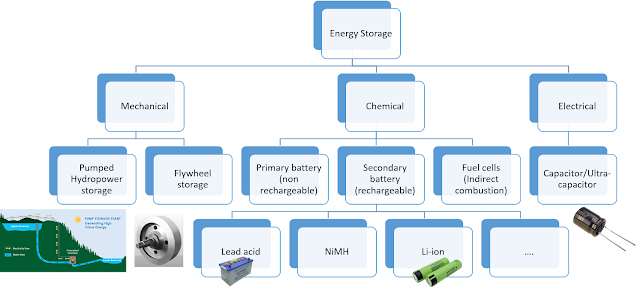 |
| Figure 1: Energy Storage Devices Family |
As can be seen in figure 1, Chemical energy devices can be roughly divided into 3 types.
- Primary Battery: Designed only for one-time use. Predominantly used in consumer electronics and some of the special purpose space applications (e.g. alkaline batteries used in TV remotes)
- Secondary Battery: Designed for multiple charge-discharge cycles. As of now, these type of devices are the most promising candidates for electric mobility.
- Fuel cells: In simple words, Fuel cell carries out indirect combustion of fuels (hydrogen or hydrocarbons, in some cases metals like Aluminum, Magnesium, etc.) Fuel cells will most probably replace secondary batteries as a power source for future mobility applications
In this blog post, we will keep our discussion limited to LiBs only.
LiB working mechanism
'Battery' Vs 'Cell'
Before we go into the operating mechanism of LiB, I want to clear out a terminology, 'battery' and a 'cell'. A 'Battery' may consist of single or multiple cells. or to be more simplistic, A battery is made up of multiple cells arranged in series and/or parallel. For further clarification, you can go through my previous blog post: Battery Pack 101
However, for this article, I will use LiB and Lithium-ion cell interchangeably for ease of use.
Lithium-ion Cell Charging and Discharging Mechanism
Figure 1 shows the basic construction of a Li-ion cell (it is not 100% correct! though easy to understand). A basic cell has two terminals (or electrodes, the positive one is called 'Cathode' and the negative terminal is called an 'Anode')
In the case of LiB, Anode consists of a graphite material (in most of the cases) and Cathode has Li-ion source materials (for example Lithium Cobalt Oxide, Lithium Aluminum Nickel Cobalt Oxide, Lithium Iron Phosphate, etc. we will see the difference between each of materials in the next blog post)
As shown in figure 2, when we connect a charger to LiB, Li+ ions get out of cathode material and get into the anode material. This process of Li+ ions getting inside anode is called 'Lithium intercalation'. When all Li+ ions intercalate into the anode, LiB is fully charged. (in practice, not all the Lithium ions from cathode intercalate into the anode. The amount of intercalation depends upon the cathode material, anode material, stability of a Lib, etc.)
 |
| Figure 2: LiB Charging mechanism |
LiB discharging mechanism is the exact opposite of the charging mechanism. As shown in figure 3, when we connect any load across LiB terminals, previously intercalated Lithium goes back to Cathode material (this is called de-intercalation).
 |
| Figure 2: LiB discharge mechanism |
When Lithium de-intercalates from the anode, it gives out an electron, converts into a Li+ ion and goes back to the cathode. Reactions at the anode can be simply put as shown in figure 4. It takes 6 carbon atom to intercalate one Lithium atom (this is theoretical maxim capacity of the graphite anode material)
 |
| Figure 4: Reactions at the Anode |
Non-Idealities in LiB Operation
Till now we have seen the ideal operation of LiB. i.e. Lithium gets out of Cathode material while charging and returns back to the cathode while discharging. However, it is not as simple as it looks! There are many non-ideal factors involved. We will look at some of the basic non-idealities
Charge-Discharge efficiency
A very idealistic picture of LiB is shown in Figures 2&3. The blue part shown in these figures is an electrolyte (I haven't talked about it yet.. consider it as a Lithium ion-conducting medium. it can be in liquid or solid phase).
When Li-ion gets out of cathode, some side-reactions of Li-ion, cathode, and an anode with electrolyte occur. These side reactions result in the irreversible loss of Li-ion. This implies the number of Li-ions got out of the cathode while charging is not the same as that of the number of Lithium ions got back to the cathode while discharging. (it may be difficult to understand. I will put it in simple words. The amount of charge got into a battery when charging is more than that of what we can get out of battery when discharging!)
The coulombic efficiency of a battery is defined as a ratio of total charge got out of the battery while discharging to the total charge got into the battery while charging. Generally, LiBs have 88-95% coulombic efficiency (much higher than lead-acid batteries).
Heat Generation
One more important non-ideality of the above shown LiB model is Heat generation. Since the anode and cathode are not ideal conductors of electrons (or electricity), when electrons flow through electrodes, heat is generated depending upon the amount of current flowing.
When currents are small heat generation is very limited, so no adverse effect on the battery. However, when a large current flows through the battery (say in case of short circuit), heat generation increases. This affects battery performance. Most of the times short-circuiting LiB terminals will result in catastrophic battery breakdown as shown in the following video on youtube.
There so many other non-idealities involved with LiBs. For example, as we keep on charging-discharging, the capacity of LiB to store energy keeps on decreasing. There are tones of degradation mechanisms. I would recommend going through following web resources for the further understanding of LiB operation and degradation mechanisms:
I have tried to cover a very basic mechanism of LiBs. In the next blog, I am planning to write about different LiB chemistries, their advantages, and their disadvantages. Please let me know if you have any questions in the comments section.
-- Harshad


Q1) I want to understand like when we provide charging current, from where the electrons originates and whats generates these electrons. Same for the discharging as well.
ReplyDeleteQ2) What is the role of voltage provided from charger and how it affects battery charging?
Hi,
Deletebrief answer yo your question:
A1> charging current itself is a flow of electrons. battery charger/power supply provides/generates those electrons.
At the time of Discharge, battery -ve terminal generates electrons. (Lithium form LiC6 converts into Li+ giving up an electron at anode i.e.-ve terminal)
A2> In actual case, battery charger doesn't control voltage, it controls charging current with upper voltage threshold equal to max charge voltage of a battery. charging voltage is regulated by battery itself. You can have a look at this page for more details:
https://batteryuniversity.com/learn/article/charging_lithium_ion_batteries